June 29, 2022 – Anitoa Systems announced on Wednesday that its PCR-based test for monkeypox has received CE...
May 5, 2022 – The company is currently working on a programme to develop tests for tropical diseases ...
January 10, 2022 – Anitoa’s solution avoids the complex process of packing and shipping patient samples to a central laboratory ...
October 27, 2021 – The Maverick line of qPCR instruments from Anitoa Systems has received U.S. Food and Drug Administration registration and device listing as class II 510(k) exempt medical devices ...
January 11, 2021 – Anitoa Systemc LLC, market leader in rapid, portable PCR-based molecular testing, has received the CE (Conformité Européene)-IVD Mark for its Maverick series portable qPCR instruments ...
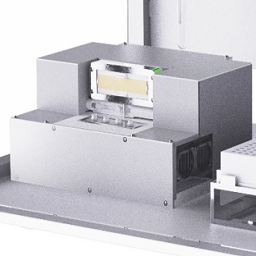
December 15, 2020 – Anitoa Systems has received the CE-IVD mark for its Maverick portable qPCR ...
March 10, 2020 – A portable on-site nucleic acid test of the 2019 novel coronavirus (2019-nCoV) analyzes patient samples ...
February 27, 2020 – Anitoa Systems, a Silicon Valley biotech company has demonstrated a solution for rapid on-site nucleic acid test of the 2019 novel coronavirus (2019-nCoV) ...
March 7, 2019 – The Anitoa sensor achieves a high level of sensitivity, which is a million times higher than the sensors in consumer digital cameras or …
March 12, 2019 – 今天在旧金山 Tri-Con 的初创公司 Anitao Systems 宣布了其4通道 qPCR 系统 Maverick 系列的 Android 移动应用程序。
February 15, 2018 – NEW YORK (GenomeWeb) – Anitoa Systems, a Silicon Valley-based medical device company, has launched an early-access program for a …
December 2016 – BioPhotonics – The ULS24 ultralow-light CMOS bio-optical sensor from Anitoa Systems LLC is a single-chip device capable of 3 × 10−6 lux low-light detection. …
Small, fast, cheap molecular diagnostics
September 1, 2015 – Startup Anitoa (Palo Alto, Calif.) described a low-cost, portable molecular diagnostics system it hopes to launch in December. The ULS24 aims to identify in less than 90 minutes a wide variety of diseases so they can be treated quickly. …
May 26, 2015 – Palo Alto–Anitoa Systemsは、中国の浙江大学と提携し、Anitoaの超微光CMOSバイオ光センサを用いたハンドヘルド定量的ポリメラーゼ連鎖反応システム(qPCR)のデモンストレーションに成功した。
November 13, 2014 – A CMOS- and LED-based imager demonstrates sensitive, specific measurement of pathogens that cause infectious diseases. Such an effective and affordable point-of-care tool promises to save lives and contain the spread of lethal and drug-resistant microbes.